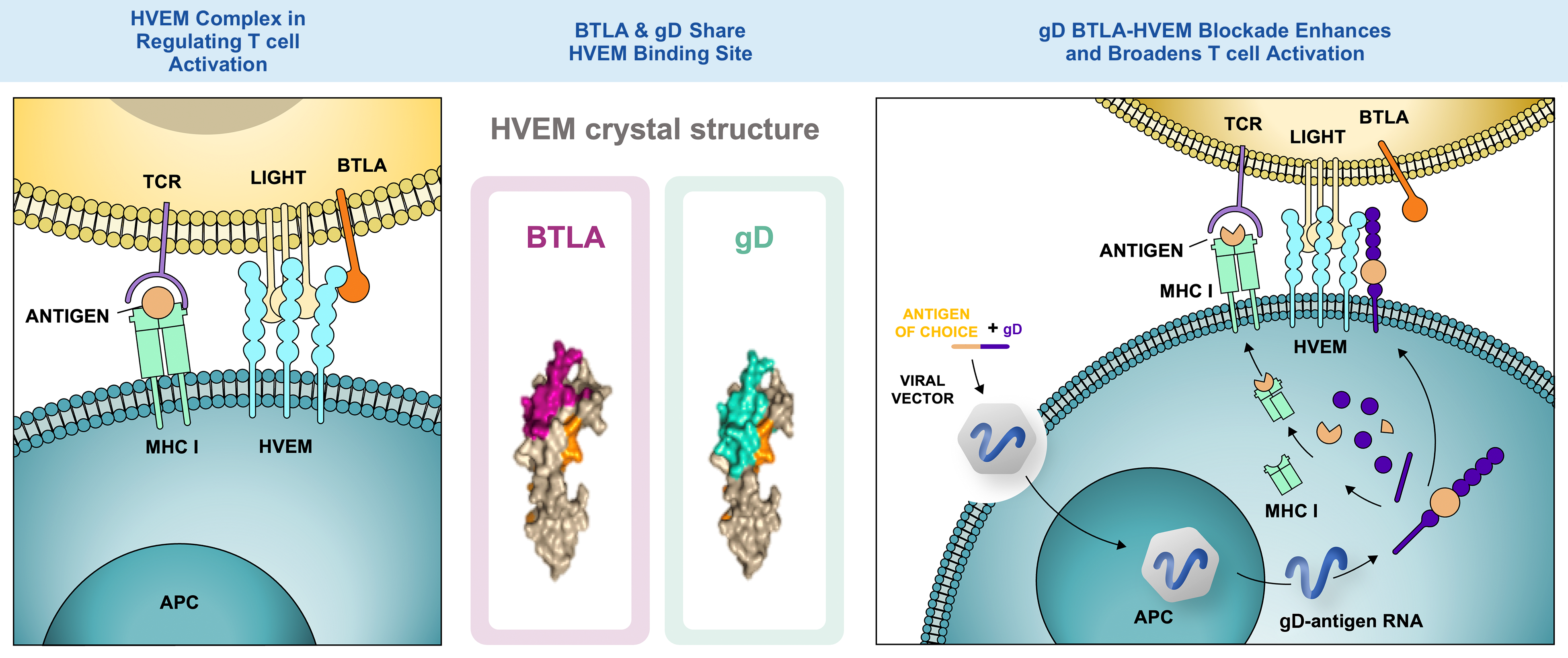
Mechanism of Action of Glycoprotein D (gD)
Herpes simplex virus gD is a genetically encoded checkpoint modifier adjuvant. It works at the site of T cell induction, promoting enhanced CD8+ T cell responses and broadened responses by recognizing subdominant epitopes that generally fail to reach the threshold for T cell activation. We believe that inducing potent, prolonged, broad, and highly functional CD8+ T cell responses can translate into novel, adaptable, and accessible therapies to fight disease, alone or in combination with other treatments.
Herpes Simplex Virus gD – The Genetically Encoded Checkpoint Modifier Adjuvant
The gD BTLA-HVEM blockade can enhance and broaden T cell activation.1–4
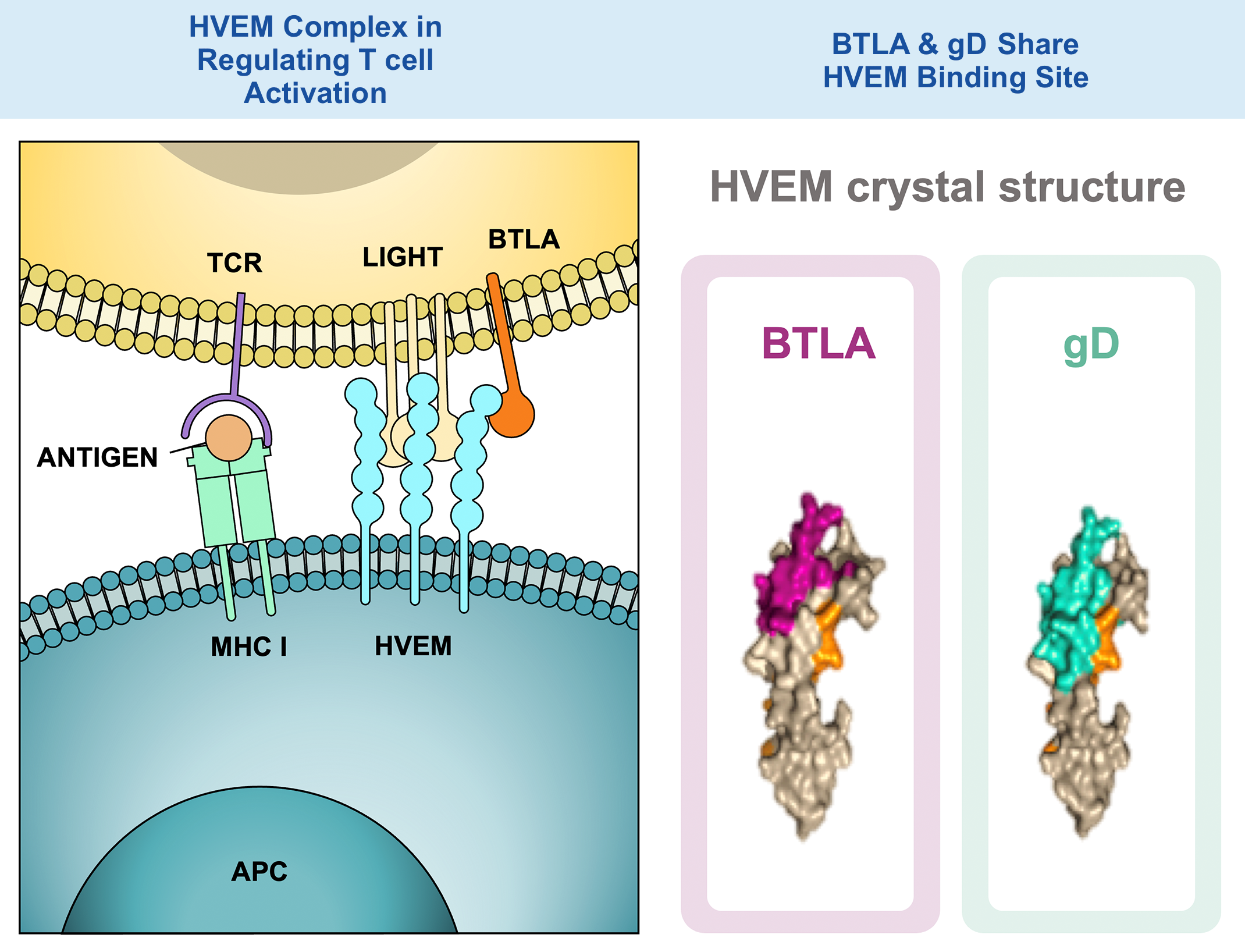
HVEM Complex in Regulating T cell Activation
1. BTLA-HVEM: inhibition
2. LIGHT-HVEM: stimulation
3. BTLA-HVEM-LIGHT ligation sends a dominant inhibitory signal to limit activation signals from TCR binding
BTLA & gD Share HVEM Binding Site
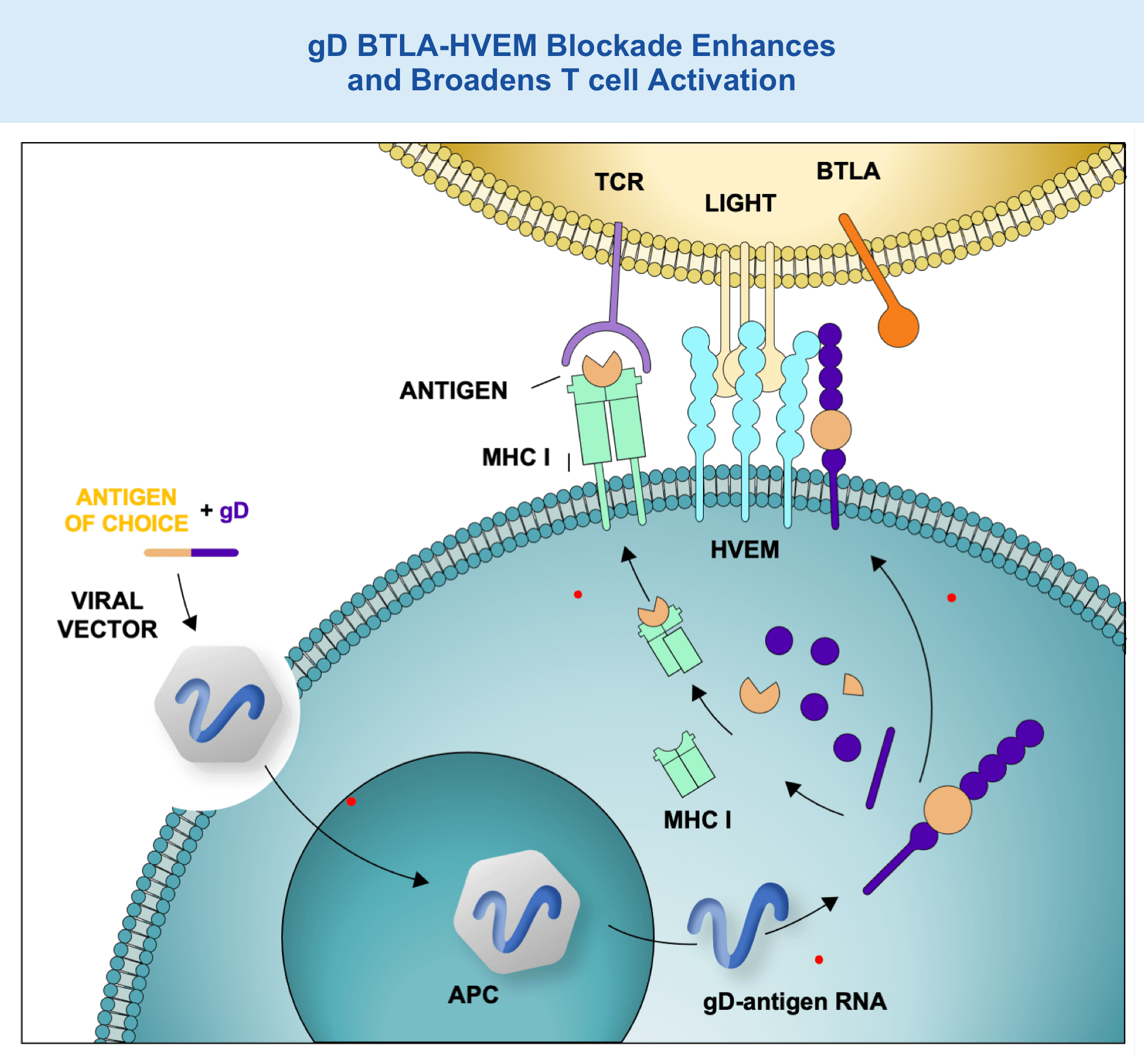
gD BTLA-HVEM Blockade Enhances and Broadens T cell Activation
4. Following intramuscular injection, VRON-infected antigen presenting cells travel to regional draining lymph nodes
5. Within antigen presenting cells, the adenovirus vector produces fusion protein of gD + antigen of choice
6. Degradation of incorrectly produced fusion protein releases peptides from the antigen, which, upon binding to MHC class I, are recognized by CD8+ T cells
7. The gD fusion protein translocates to the cell surface, where it blocks BTLA-HVEM interaction, thereby increasing T cell receptor signaling and allowing for co-stimulation through LIGHT
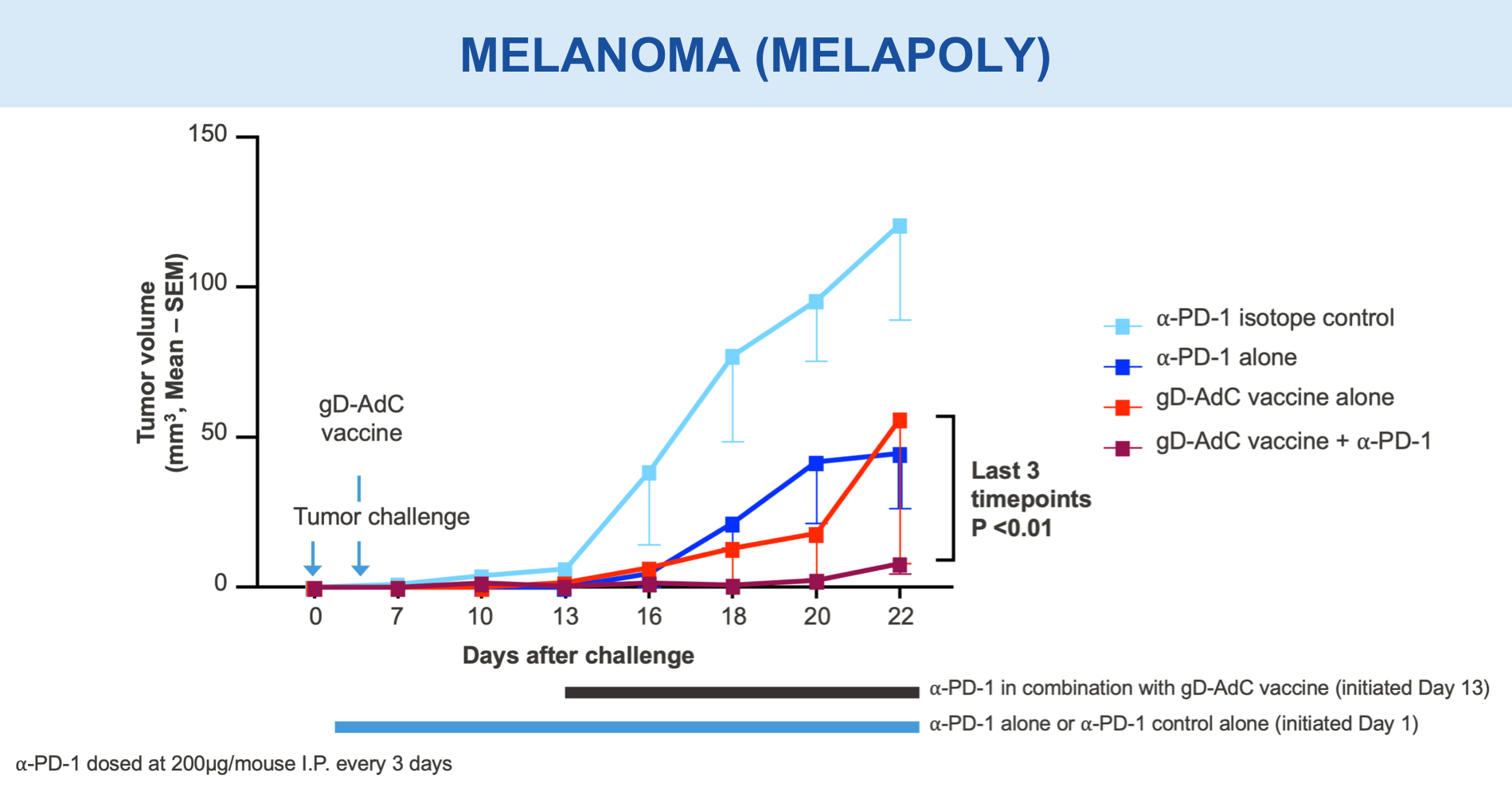
Anti-PD-1 Monoclonal Antibody Plus gD-containing Combination Demonstrated Delayed Tumor Growth
Benefits of gD’s Mechanism of Action
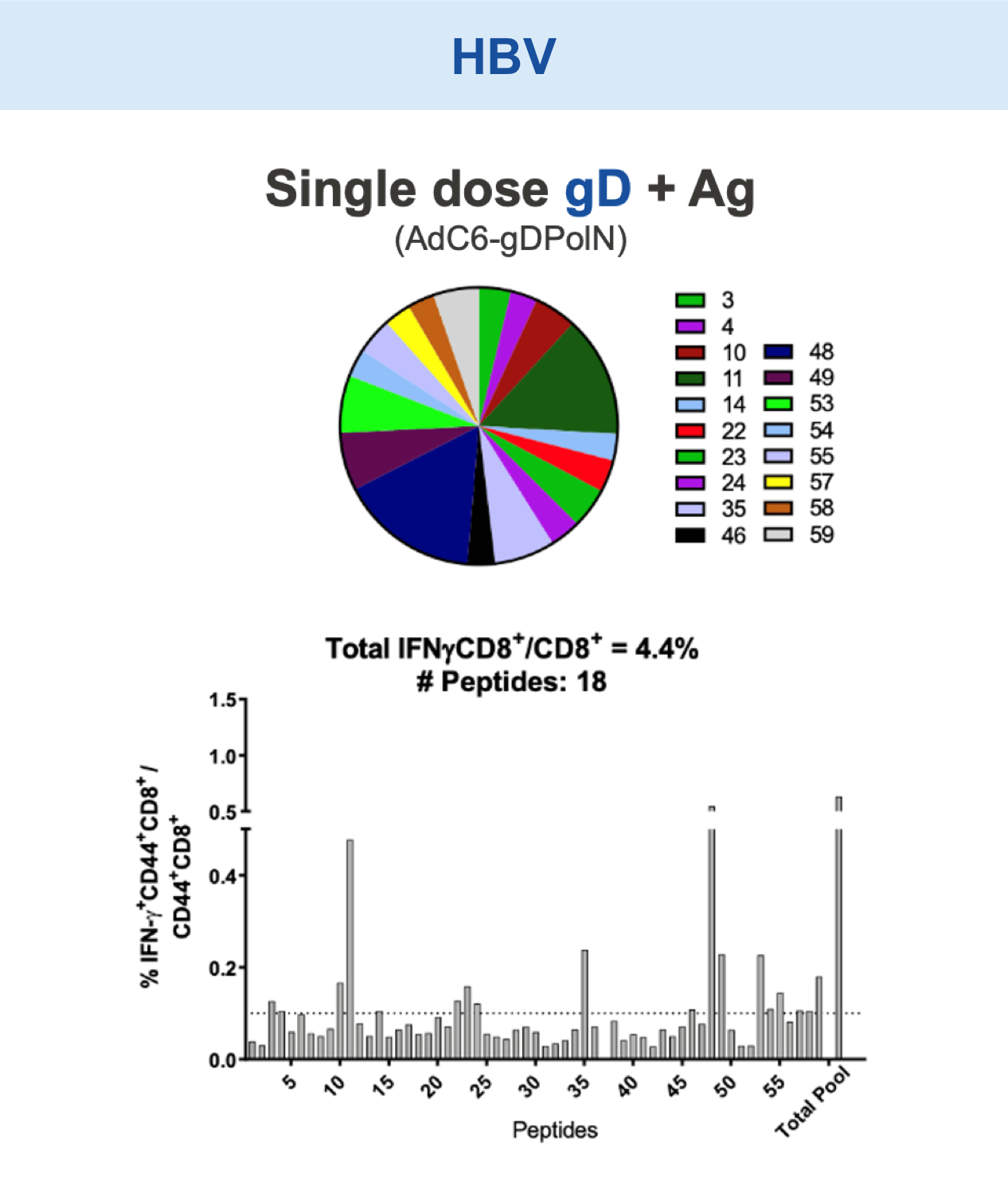
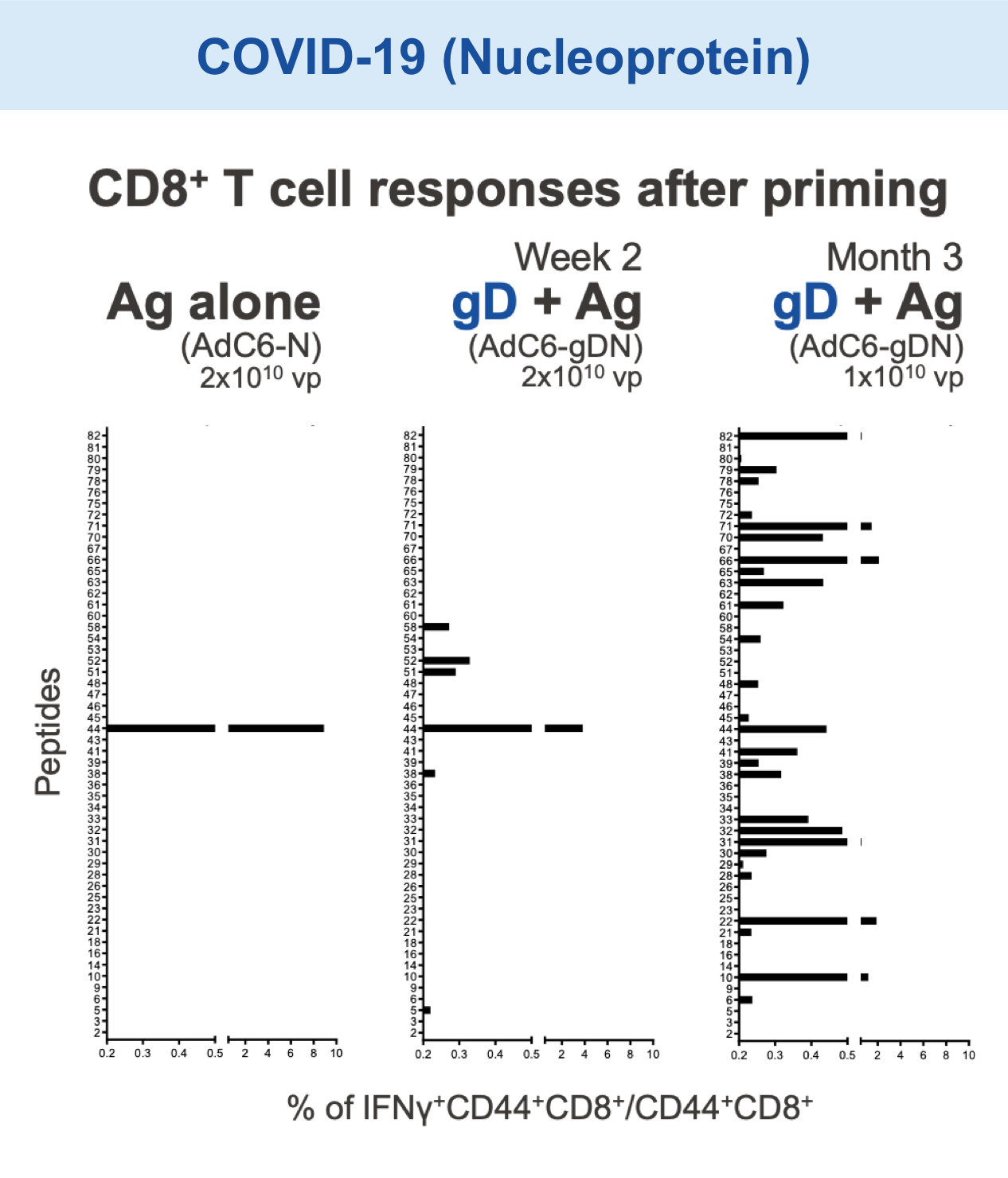
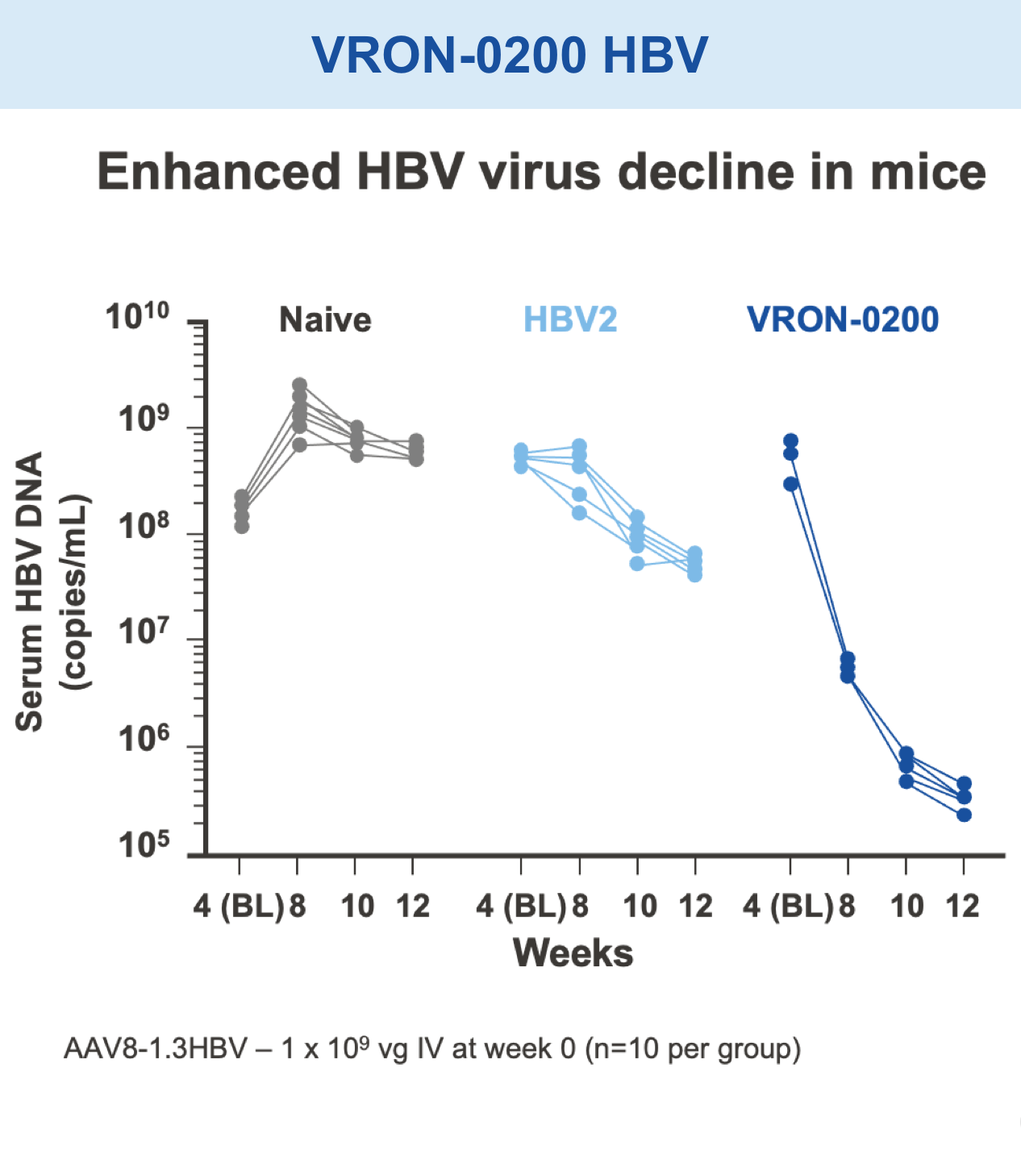
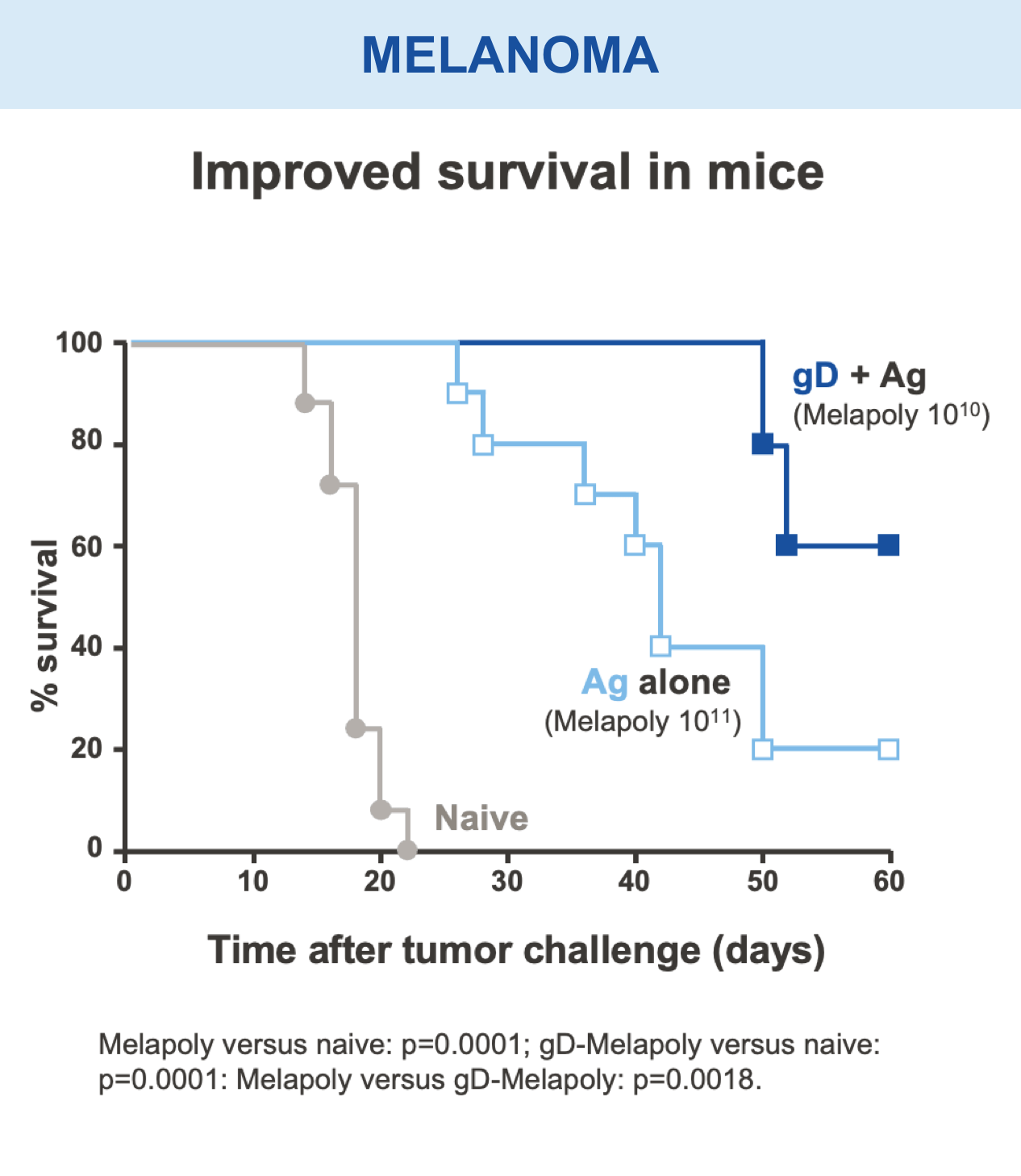
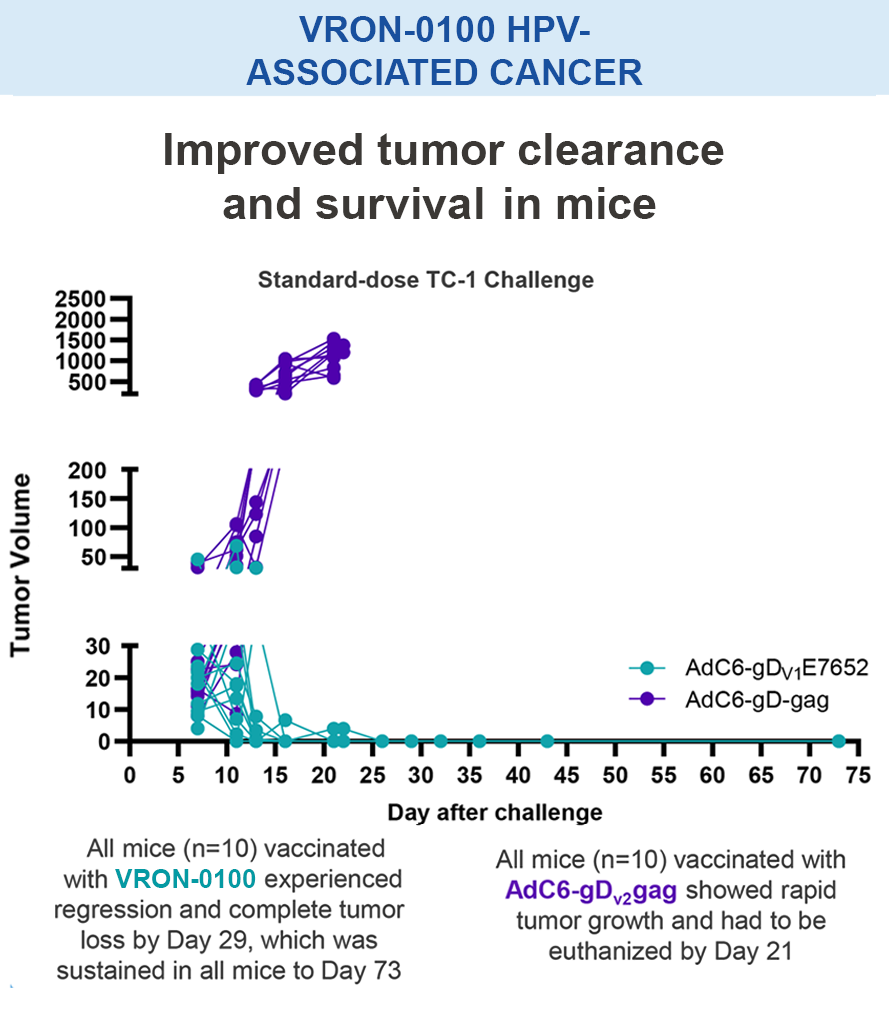
Preclinical studies in HIV, HBV, HCV, malaria, COVID-19, ebola, and in certain cancers (i.e. melanoma) have demonstrated the safety and immunogenic benefits of gD.6–10 This includes data demonstrating that gD amplifies and broadens CD8+ T cell responses to different disease-specific antigens.6–10 gD has shown promising preclinical efficacy, not only in our anti-HBV immunotherapy (VRON-0200), but also in cancers associated with HPV and melanoma.1,2,11,12 It is believed to have lower risk of “off target” side effects.

Checkpoint modification by gD lowers the CD8+ T cell activation threshold.
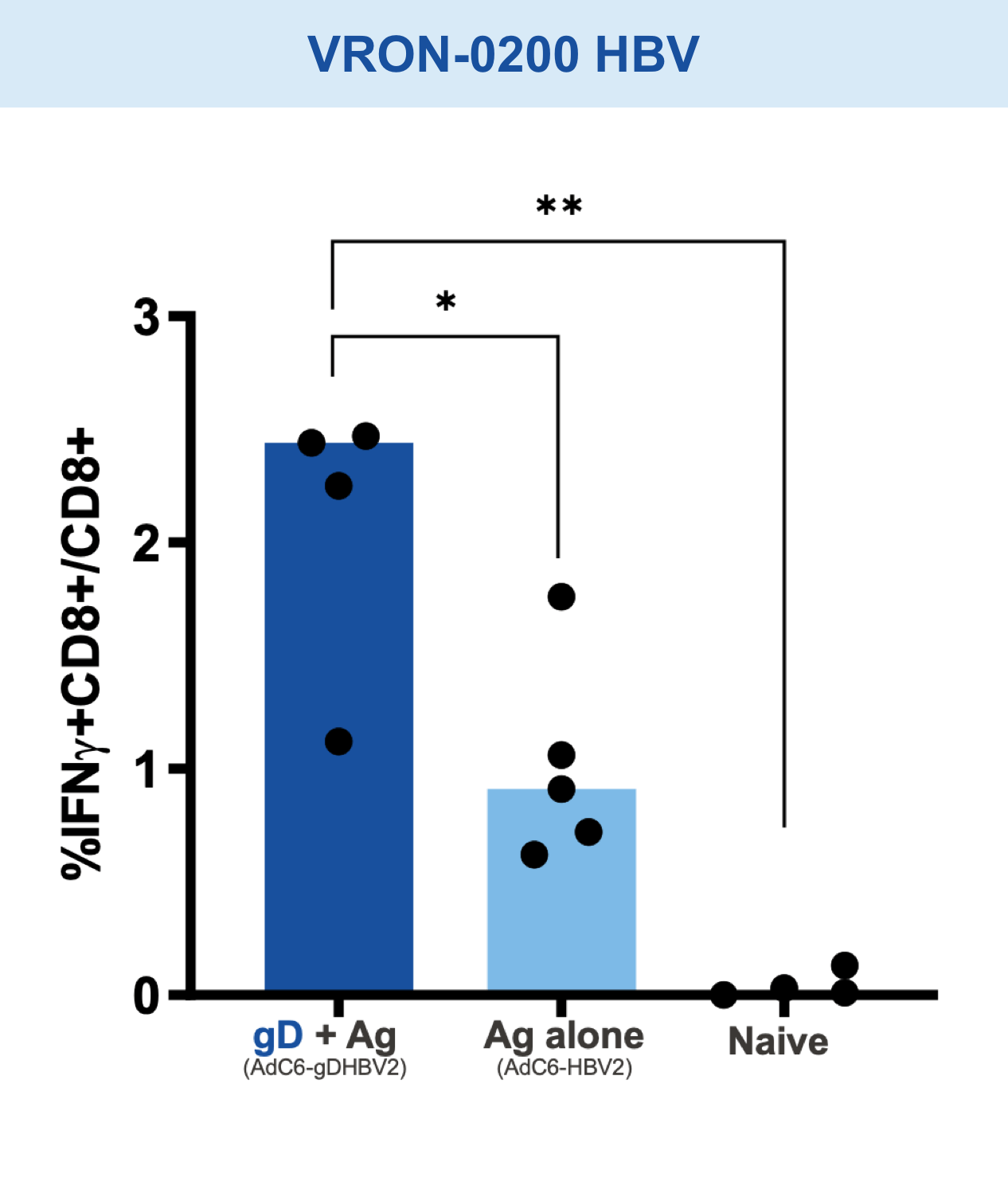
Checkpoint Modification (gD): Amplifies CD8+ T cell Responses
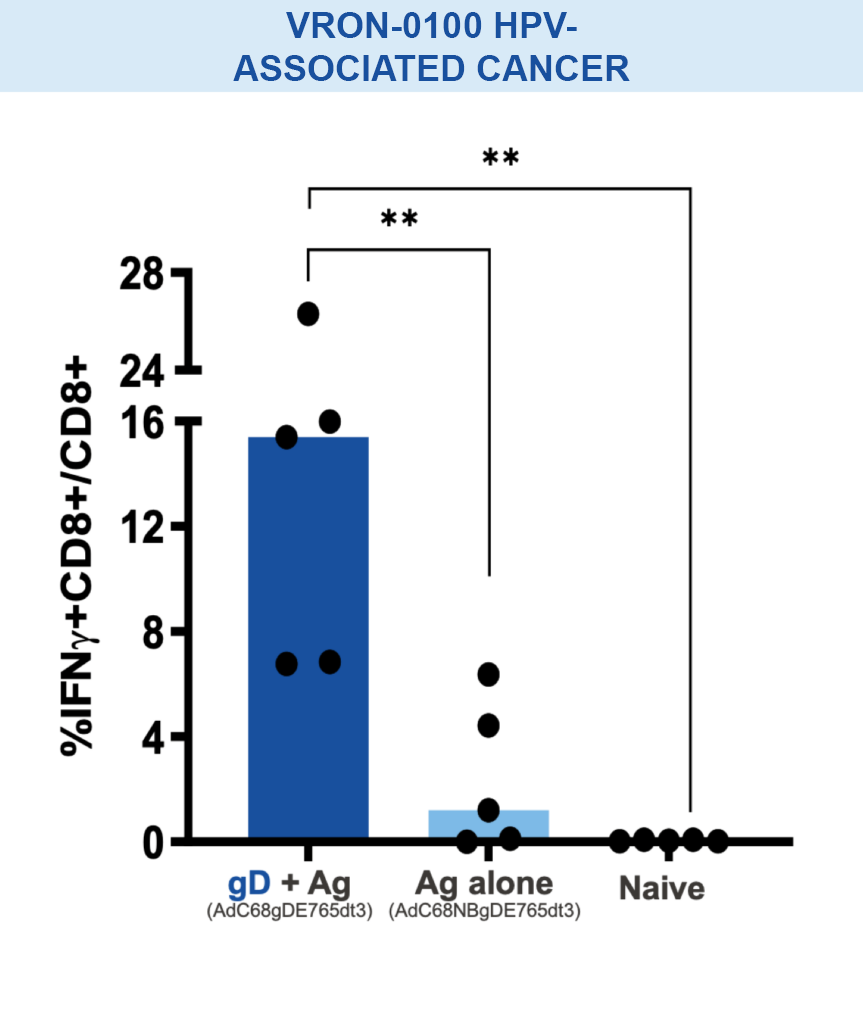
Our VIACT™ platform includes the checkpoint modifier gD plus a disease specific antigen insert. gD plus the antigen insert improved immunogenicity in animal models by amplifying CD8+ T cell responses compared with the antigen insert alone and controls.1,2,11,12
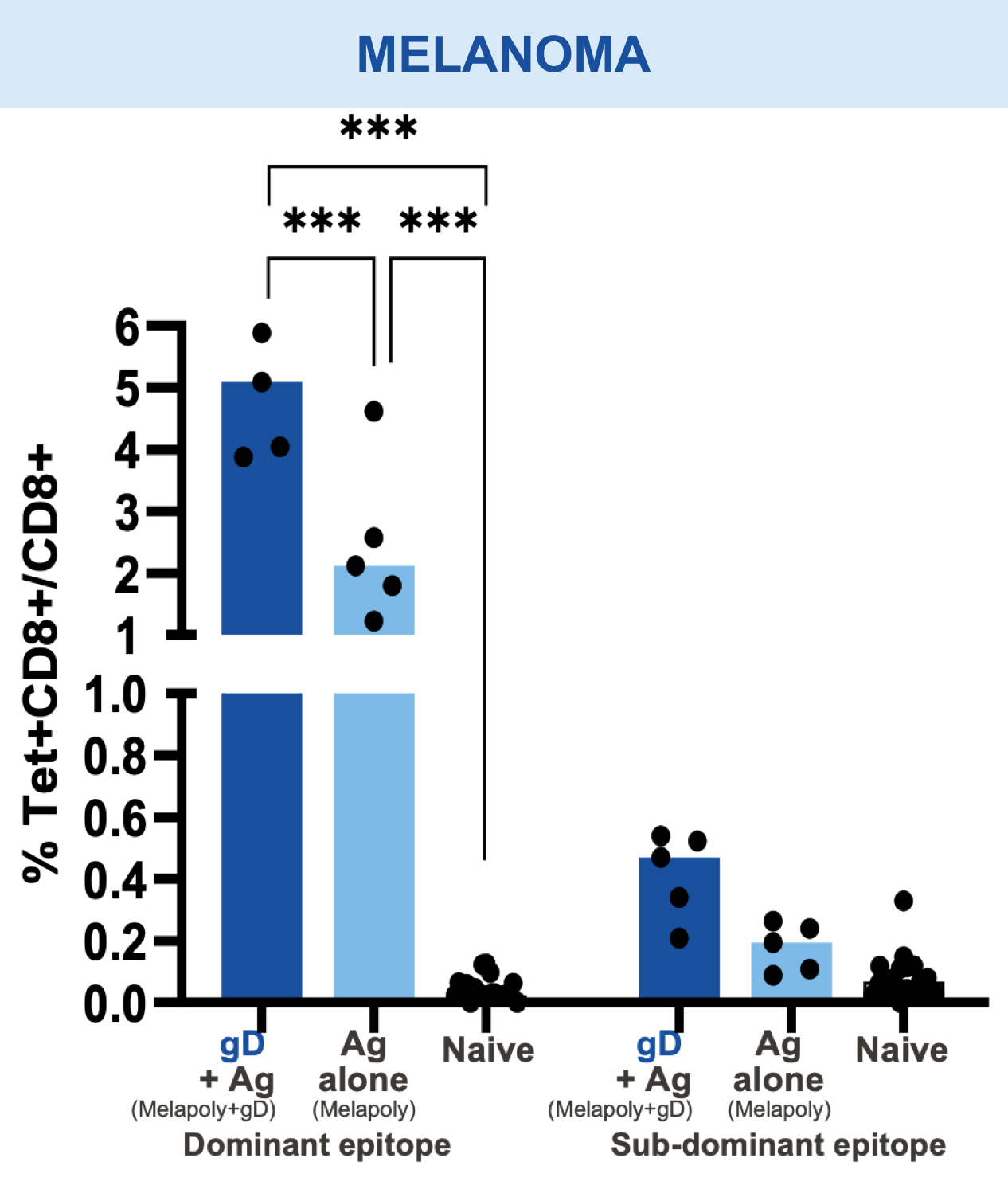
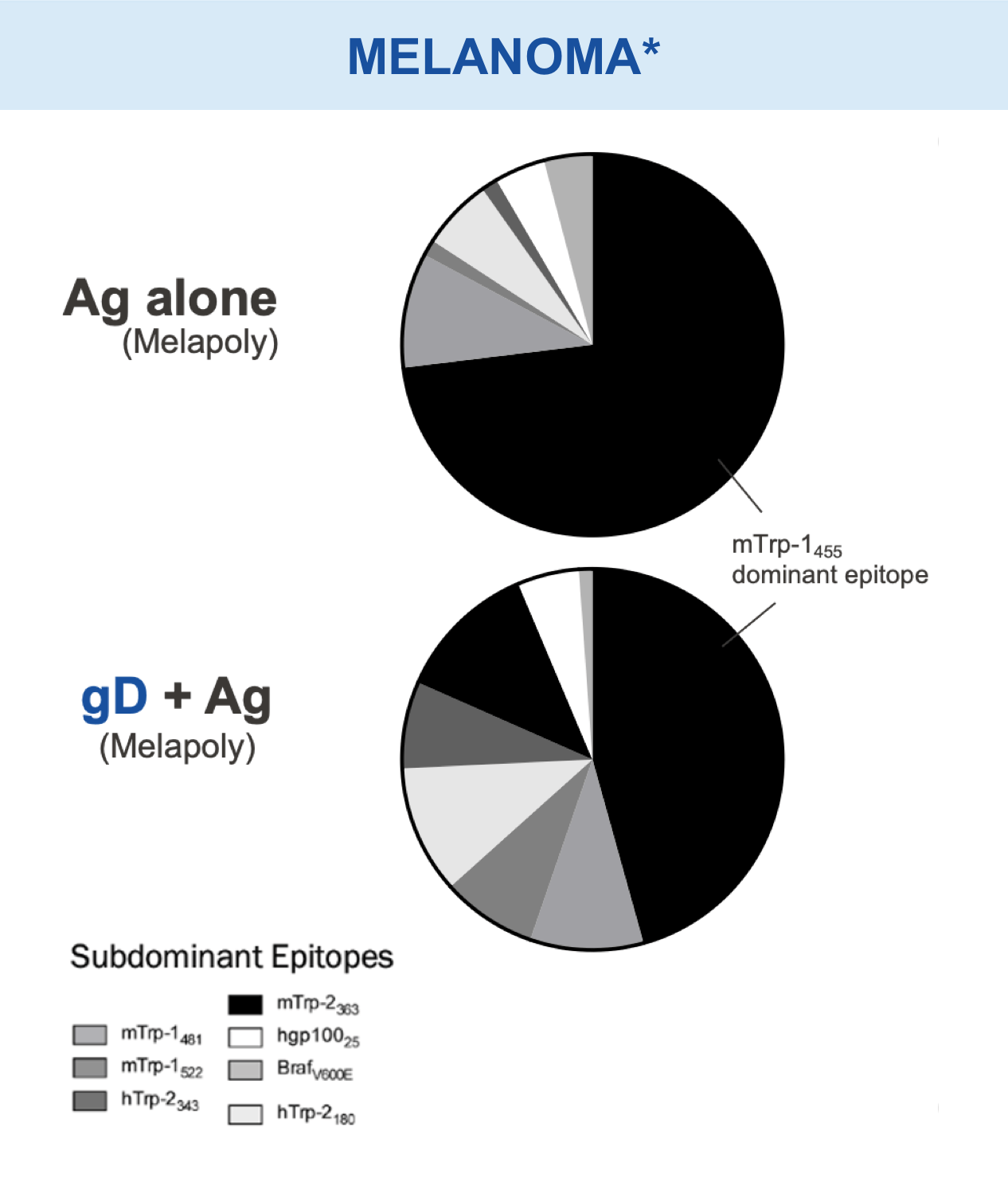
Checkpoint Modification (gD): Broadens CD8+ T cell Responses
Animal models of cancer and infectious disease showed that gD plus an antigen insert induced a broad antigen-specific CD8+ T cell response versus treatment with gD alone.1,11,13,14
Checkpoint Modification (gD): Improved Efficacy
Mice vaccinated with VRON-0200 HBV, a melanoma construct containing gD, or VRON-0100 HPV, delivered by ChiVax™ showed improved antiviral and antitumor/survival benefits over those animals immunized using the same construct without gD or a control vaccine.1,11,12,15,16
AdC, chimpanzee adenovirus serotype 68; AdC6, heterologous chimpanzee adenoviral viral vector of serotype 6; Ad, adenovirus; Ag, antigen; ANOVA, analysis of variance; APC, antigen presenting cell; BL, baseline; BTLA, B-and T-lymphocyte attenuator; CD, cluster of differentiation; E7, HPV E7 oncoprotein; gD, glycoprotein D; HBV2, HBV core & pol; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; hTrp, human tyrosinase-related protein; HVEM, herpes virus entry mediator; IFN, interferon; IL, interleukin; I.P., intraperitoneal injection; IV, intravenous; LIGHT, lymphotoxin-like, exhibits inducible expression, and competes with herpes simplex virus glycoprotein D for HVEM, a receptor expressed by T lymphocytes; Melapoly, melanoma antigens (Trp-1, Trp-2, gp100, mutated BRAFV600E); MHC, major histocompatibility complex; mTrp, mouse tyrosinase-related protein; PD-1, programmed cell death protein-1; PolN, N terminus of polymerase; SEM, standard error of mean; TCR, T cell receptor; tet, tetramer; TNF, tumor necrosis factor; Trp, tyrosinase-related protein; VRON-0200, gD fused to HBV core & pol.
- Luber A, et al. ESMO TAT 2021:Abstract 143.
- Xiang ZQ, et al. ASCO-SITC Clinical Immuno-Oncology Symposium 2020:Abstract 71.
- Stiles KM, et al. J Virol. 2010;84:11646–60.
- Virion Therapeutics. Data on file.
- Zhang Y, et al. Cancer Cell. 2017;32:377–91.
- Barnes E, et al. Sci Transl Med. 2012;4:115ra1.
- Ledgerwood JE, et al. N Engl J Med. 2014; 376:928–38.
- Hayton E-J, et al. PLoS One. 2014;9:e101591.
- Tiono AB, et al. PLoS One. 2018;13:e0208328.
- Guo J, et al. Hum Vaccin Immunother. 2018;14:1679–85.
- Zhang Y and Ertl HCJ. J Immunol. 2014;193:1836–46.
- Hasanpourghadi M, et al. EASL 2021:Abstract OS-2478.
- Hasanpourghadi M, et al. Virol J. 2021;18:242.
- Novikov M, et al. BioRxiv. Published online January 1, 2022. DOI: 10.1101/2022.02.23.481620.
- Lasaro M, et al. Mol Ther. 2011;19:1727–36.
- Currie S, et al. ESMO TAT 2023. Poster #36P.