Where Is The Unmet Need?
Despite HBV Preventative Vaccines, Chronic Infection is a High Unmet Need, with 1 in 4 People Infected, Dying Prematurely
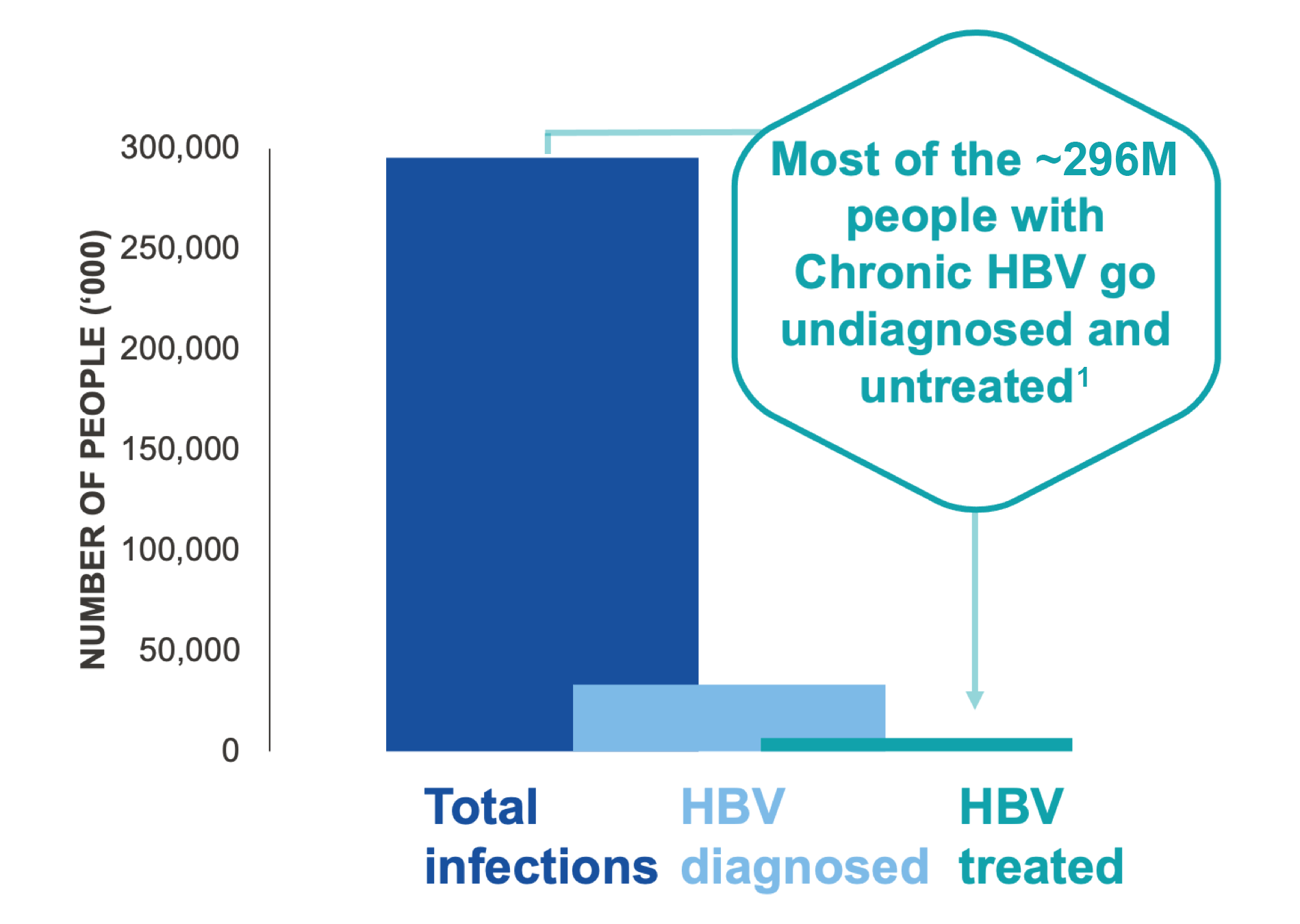
Globally, 296 million people were estimated to be infected with HBV in 2019.1 HBV infection impairs CD8+ T cells resulting in the loss of viral control. This makes HBV an ideal target for immune modulators, though therapies using currently available technology have shown limited long-term clinical benefits. There is an urgent unmet need for effective therapies that stimulate new functional CD8+ T cells and restore viral control, to address the goal of functional cure for persons with chronic HBV.

Chronic HBV remains a global public health problem despite vaccination2

1 in 4 people with chronic HBV will die prematurely from liver cirrhosis, HCC, or liver failure3
Antivirals rarely achieve functional cure and require lifelong drug therapy4

CD8+ T cells become impaired during chronic HBV infection, resulting in loss of viral control

Immune modulators for functional cure of chronic HBV have shown limited clinical benefits5–9
Novel treatments are needed to stimulate new functional CD8+ T cells
Vaccine approaches that stimulate naïve T cells to de novo epitopes may restore viral control10
OUR APPROACH FOR CHRONIC HBV FUNCTIONAL CURE
ELIMINATE
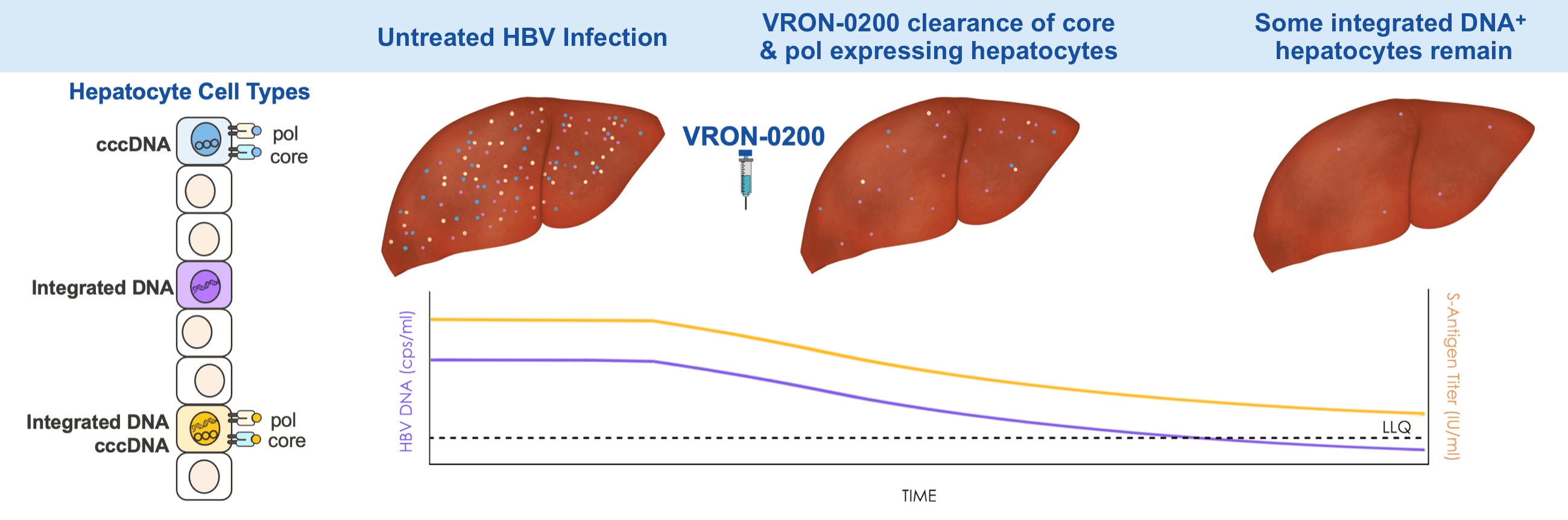
cccDNA-infected hepatocytes
STIMULATE & EXPAND
NEW HBV-specific CD8+ T cells to RESTORE immune control
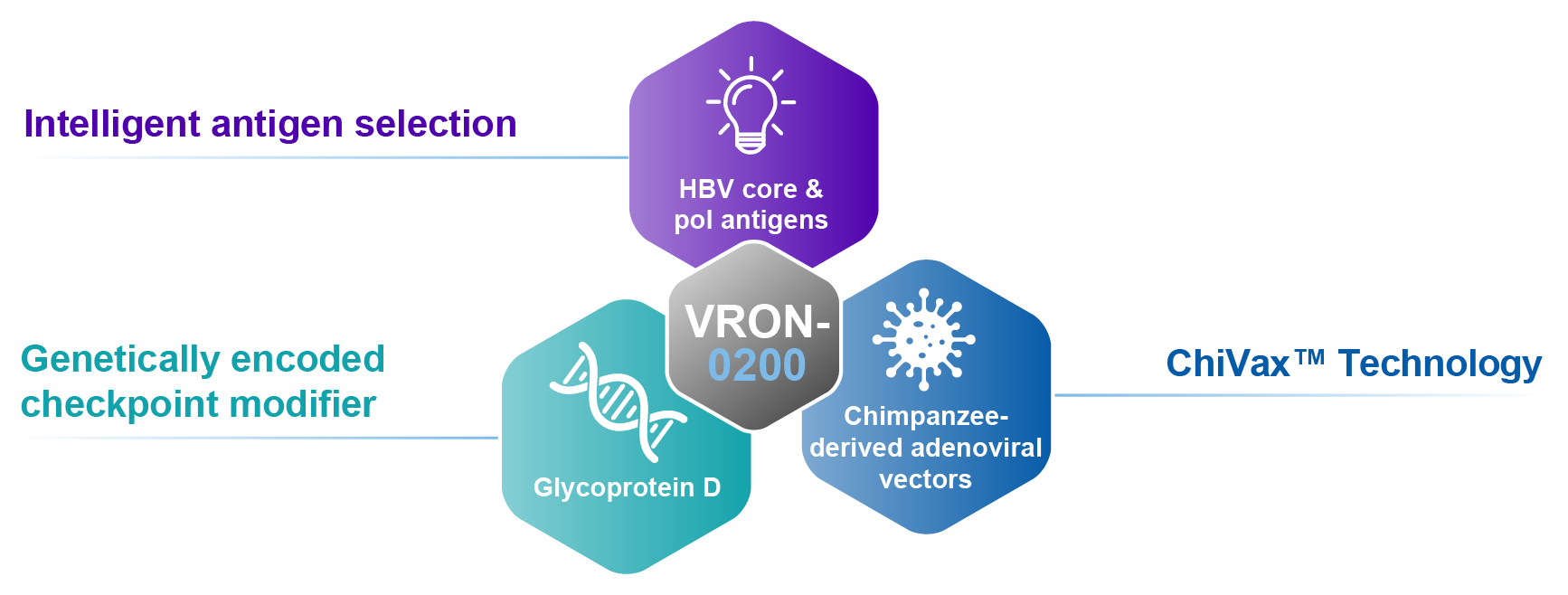
How Does VRON-0200 Work?
VRON-0200 is designed with the goal of providing a functional cure for chronic HBV infection. While the virus itself stimulates expansion of HBV-specific CD8+ T cells, they soon become exhausted, placing limits on their ability to proliferate and control the virus.11 VRON-0200, through checkpoint modification, is able to mitigate this exhaustion by lowering the activation threshold and stimulating T cells to sub-dominant epitopes that are not activated by HBV infection, which promotes further CD8+ T cell expansion and viral control.12–17
Checkpoint modification by gD lowers the CD8+ T cell activation threshold.
Addresses a global high unmet medical need
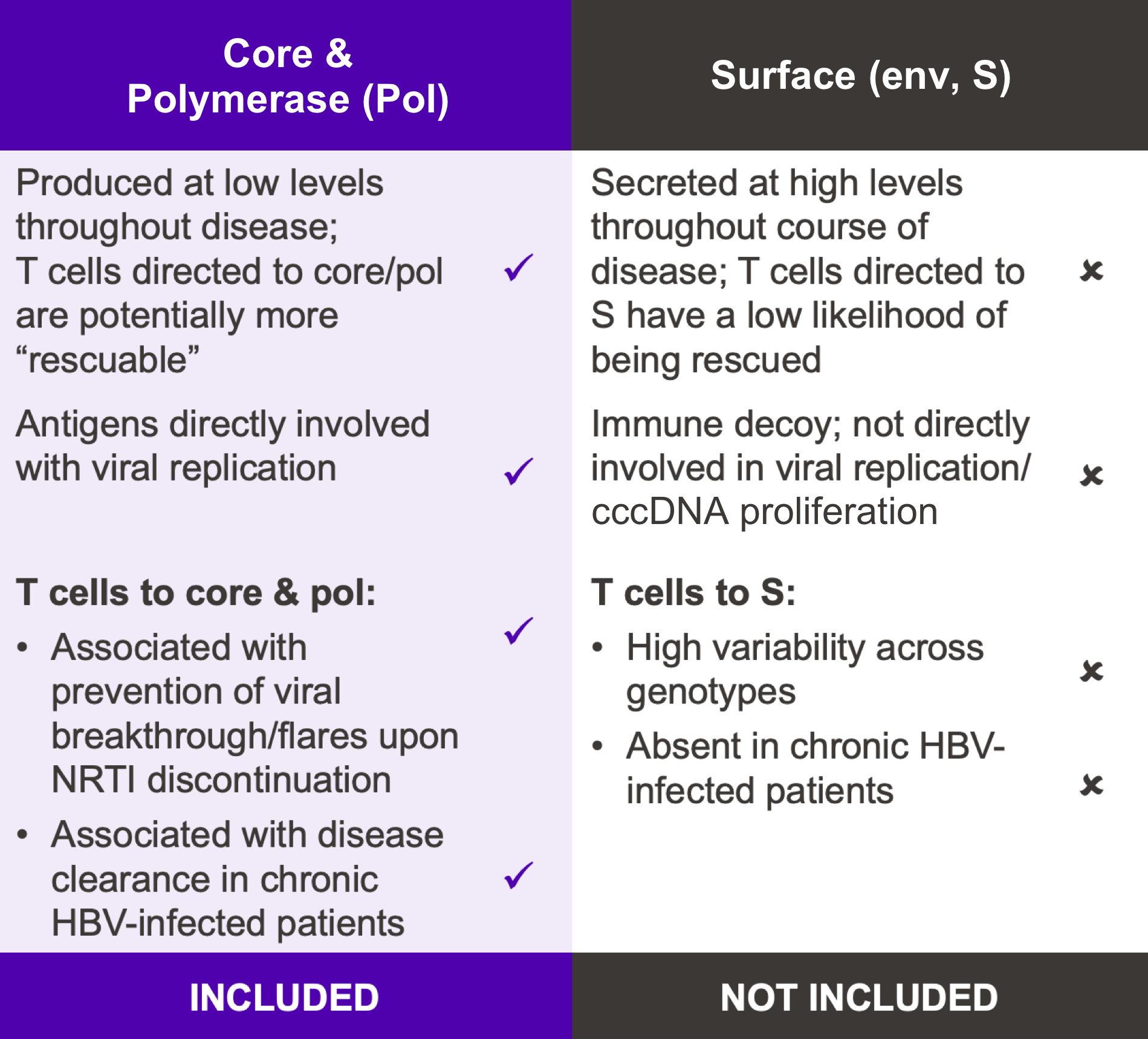
Unique design for multiple HBV functional cure programs using selected HBV core & pol antigens
HBV T cell immunotherapy with checkpoint modifier
- Amplified & expanded immunogenicity of CD8+ T cell responses
- Required for antiviral activity
- By mechanism, may lower risk for serious “off-target” side effects
Heterologous chimpanzee adenoviral vectors
- Limited pre-existing vector immunity
- Limited cross-vector immunity
- Allows for optimal prime/boost strategies
VRON-0200: Targeted Antigen Selection
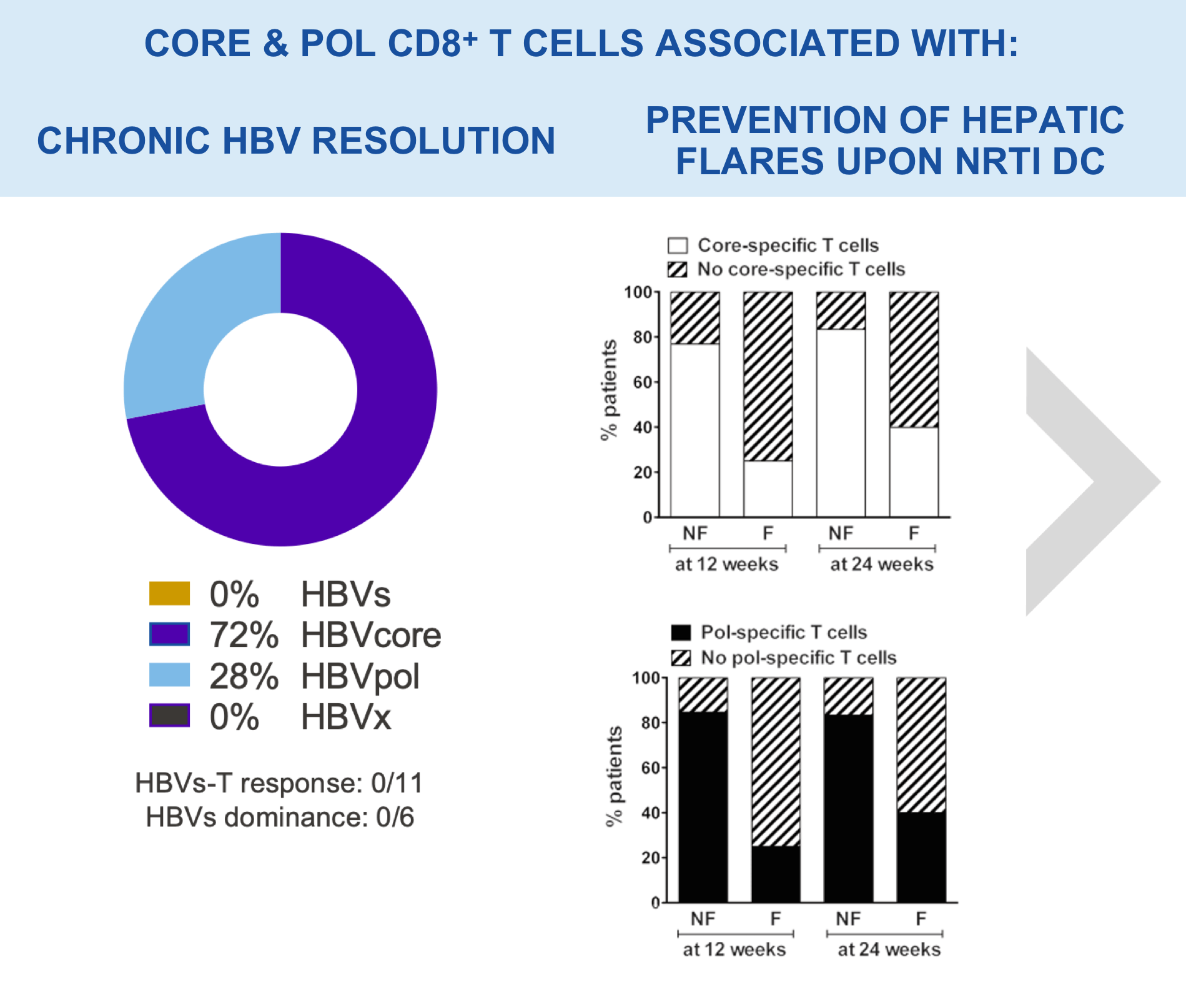
Our goal is to develop a functional cure for chronic HBV infection. Research has shown that expansion of HBV-specific CD8+ T cells induced by the infection is limited by CD8+ T cell exhaustion. VRON-0200 induces a very potent and broad CD8+ T cell response that includes responses to the core and pol regions not normally induced by the infection; as such, a new and highly functional immune response is stimulated to help clear the virus.12–14
HBV DNA & S antigen Titers Produced By Different Hepatocyte Cell Types
VRON-0200: Antigen Selection & Testing
The selection of components for our VIACT™ platform involved screening a total of 8,629 HBV viral genomes for consensus sequences across genotypes A through D. Discrepancies were adjudicated via HLA prediction algorithms for broadest possible human responses.
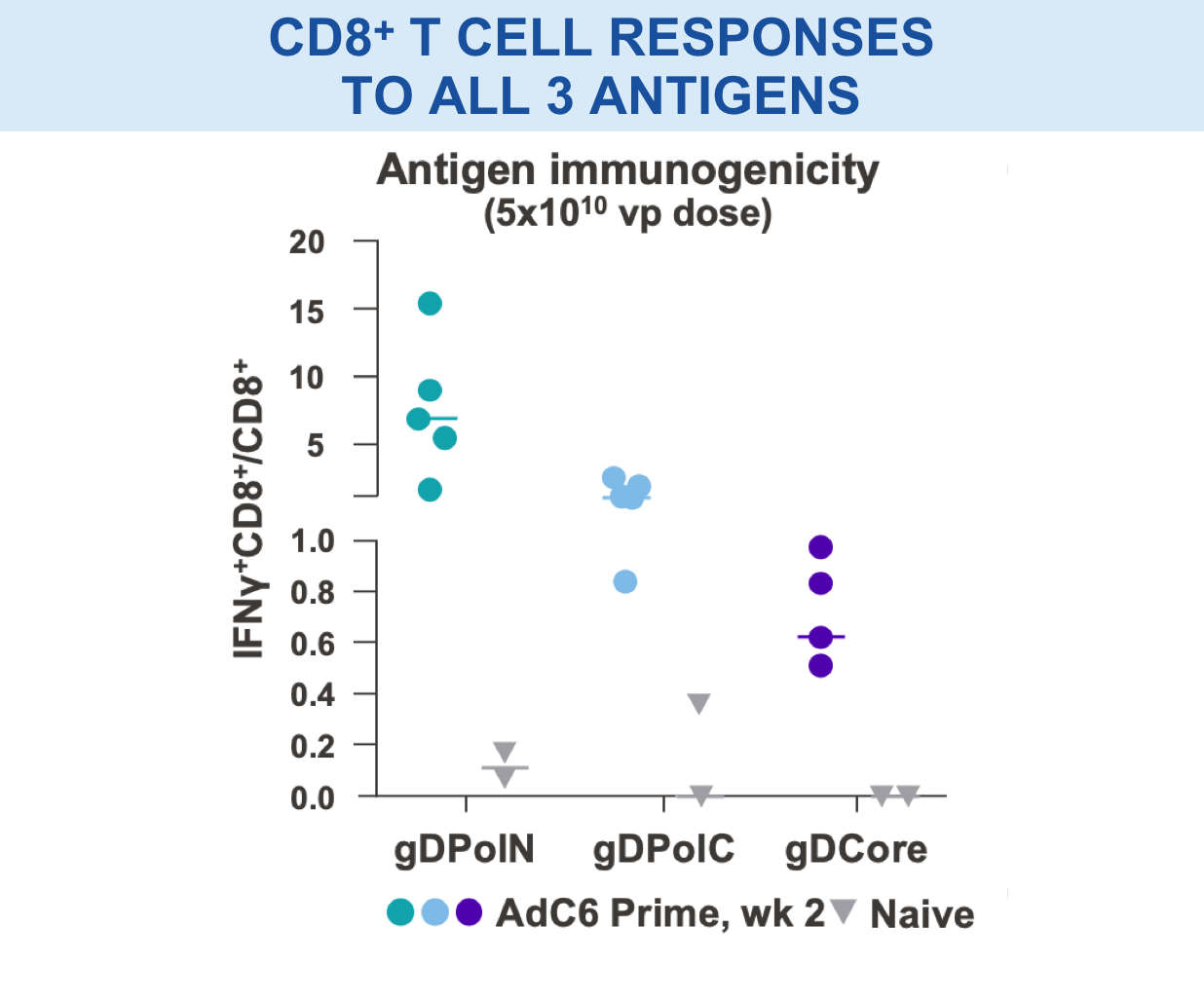
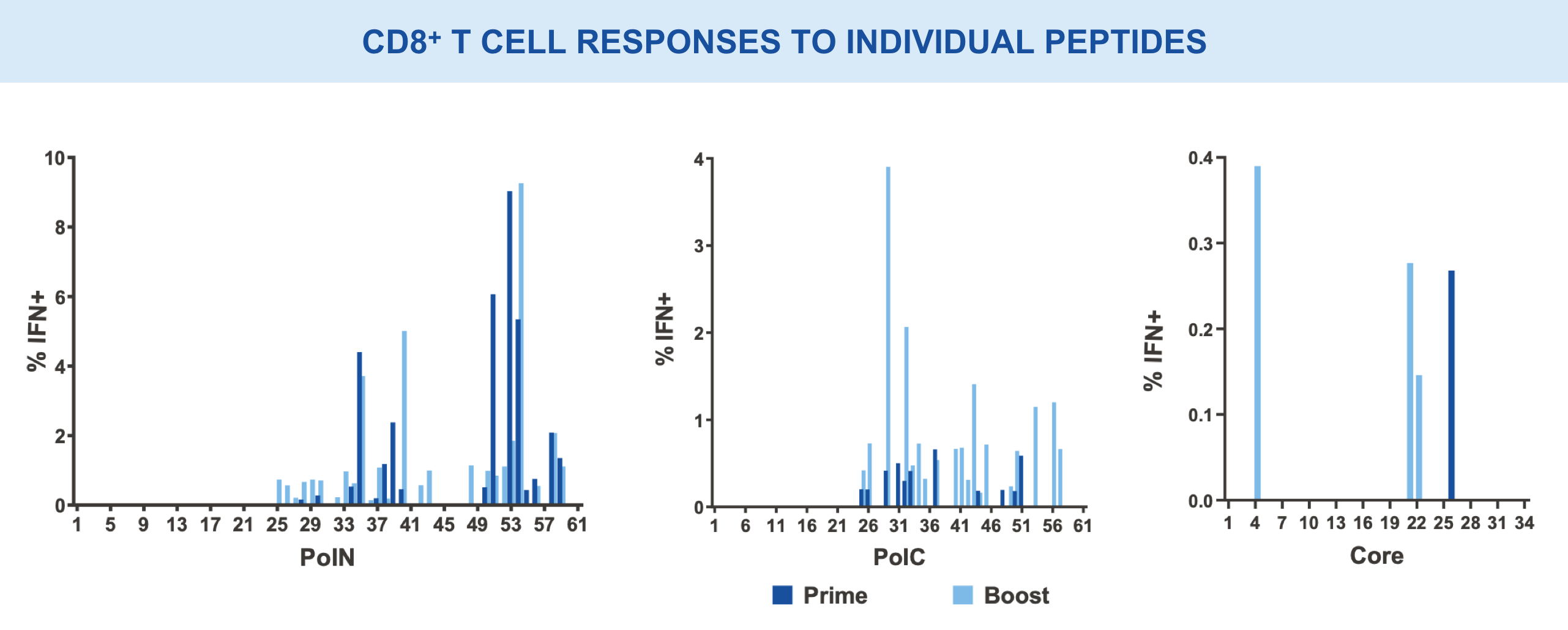
Individual regions tested separately for immunogenicity and breadth of responses15,16
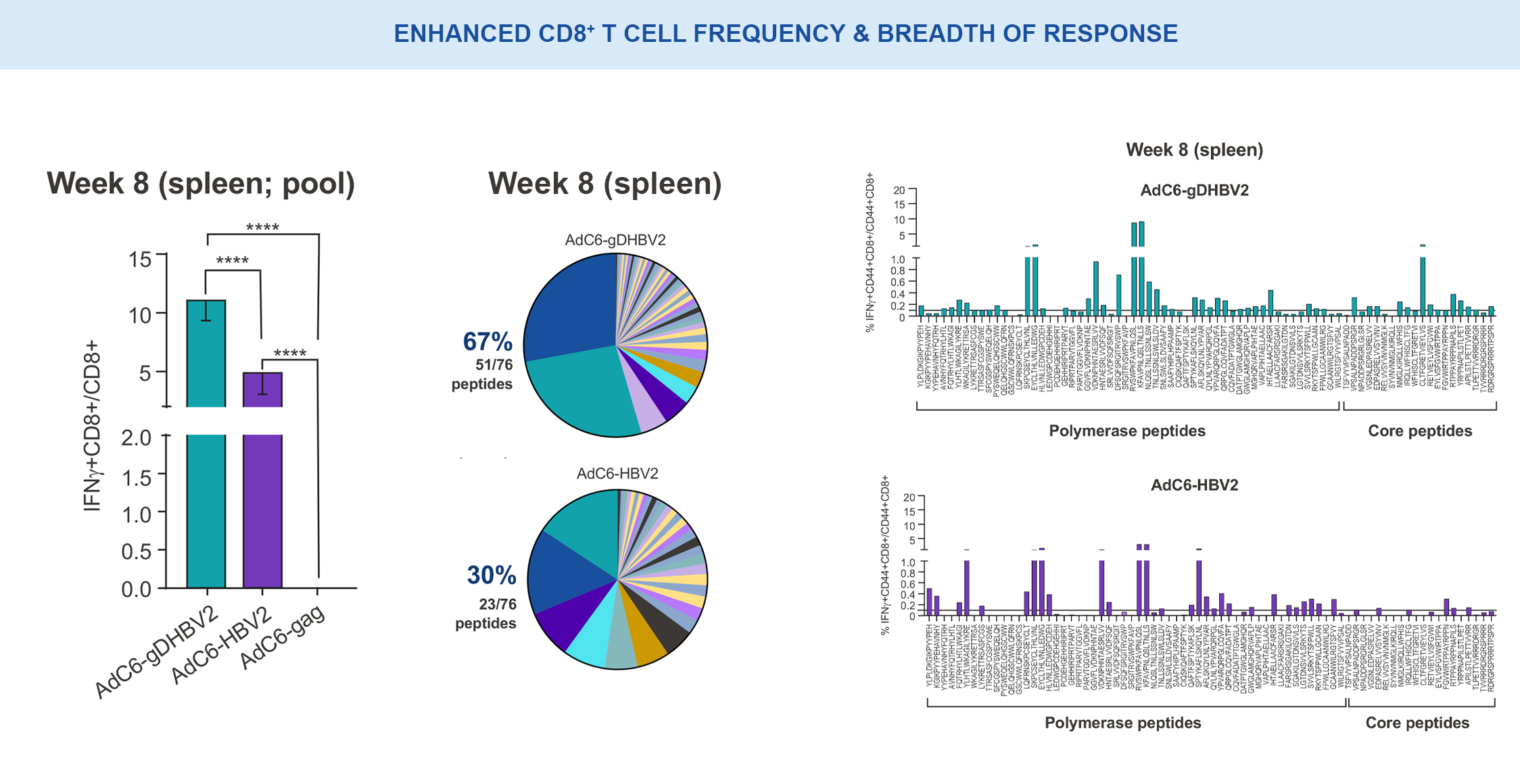
VRON-0200: Enhanced Frequency & Breadth of Response
Significantly enhanced frequencies of vaccine-induced IFNγ-producing CD8+ T cells, as well as a doubling breath of responses in spleens, in VRON-0200 treated animals.18
****p-values <0.0001 via 2-way ANOVA.
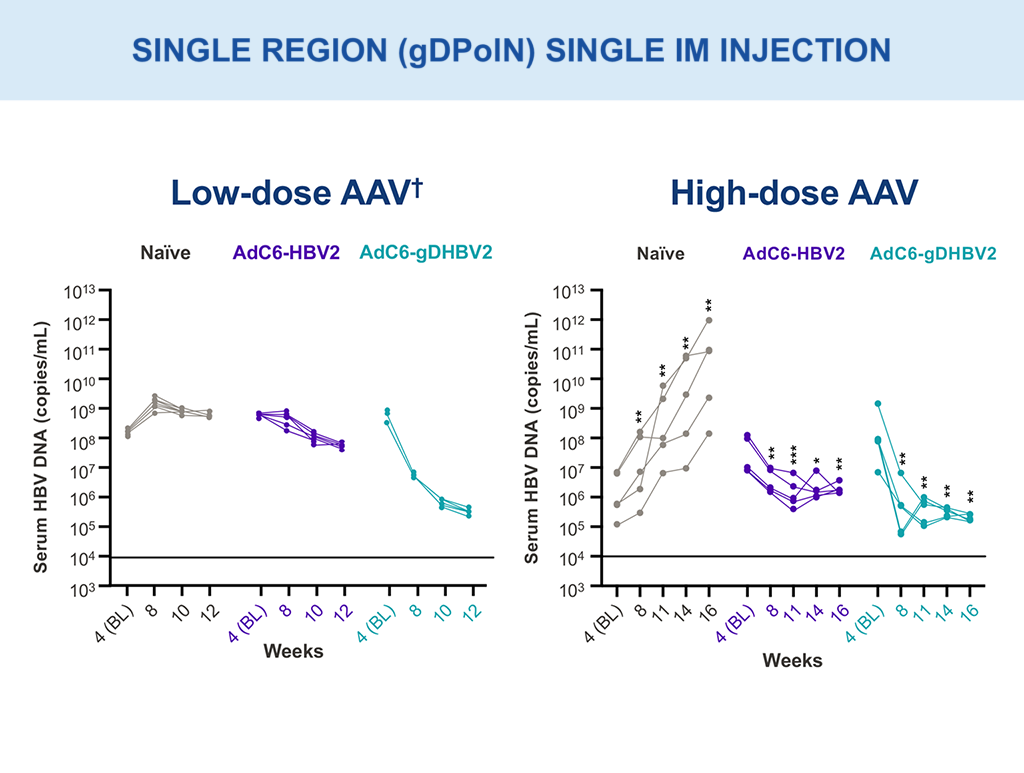
VRON-0200: Efficacy Evaluation
Multi-log HBV DNA viral load declines were observed with single intramuscular injection (AAV; gDPolN).10,18
Low dose – 1×10^9 vg; High dose – 1×10^10 vg.
**p-values between 0.001–0.01; ***p-values between 0.0001–0.001 via two-way ANOVA.
†Presented in part: EASL 2021: Abstract OS-2478.10
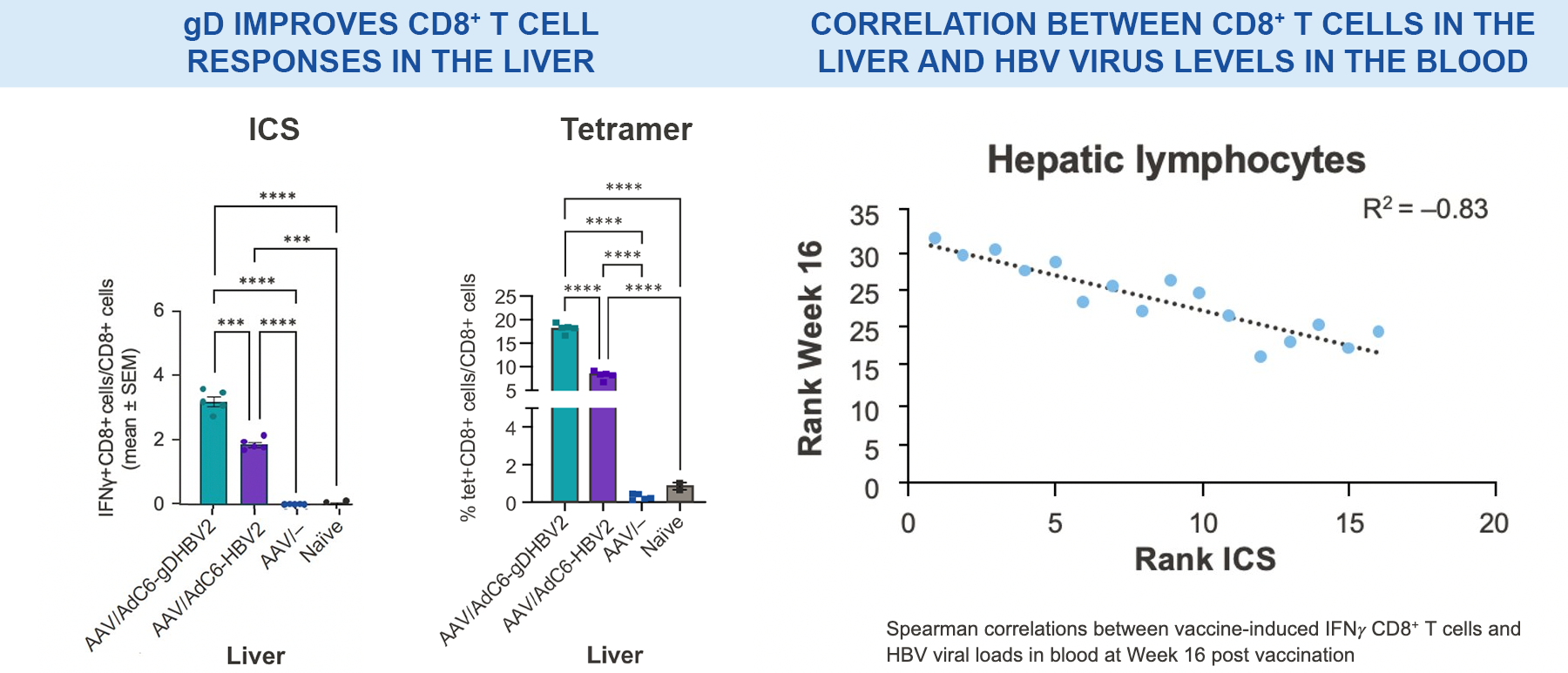
Significantly higher vaccine-induced CD8+ T cells were observed in the livers of VRON-0200 treated animals than those receiving vaccination without gD; this was found to be strongly inversely correlated with HBV DNA viral load measurements in blood.18
***p-values between 0.0001–0.001; ****p-values <0.0001 via 2-way ANOVA.
NB. AdC6-gDHBV2 is VRON-0200; AdC6-HBV2 is the same construct without gD.
AAV, adeno-associated virus; AdC6, heterologous chimpanzee adenoviral viral vector of serotype 6; ANOVA, analysis of variance; BL, baseline; cccDNA, covalently closed circular DNA; CD, cluster of differentiation; F, flare; gD, glycoprotein D; HBV, hepatitis B virus; HBV2, core & pol without gD; HCC, hepatocellular carcinoma; HLA, human leukocyte antigen; ICS, intracellular cytokine staining; IFN, interferon; IM, intramuscular; NF, no flare; NRTI, nucleos(t)ide reverse transcriptase inhibitor; pol, polymerase; PolN, N terminus of polymerase; PolC, C terminus of polymerase, vg, viral genomes; vp, viral particles; VRON-0200, core and pol fused with gD.
- WHO Hepatitis B Fact Sheet, 18 July 2023.
Available: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Accessed September 2023. - Bertoletti A, et al. J Hepatol. 2016;64(1 Suppl):S71–83.
- Lok ASF, McMahon BJ. Hepatology. 2009;50:661–2.
- Tsounis EP, et al. World J Gastroenterol. 2021;27:2727–57.
- Boni C, et al. Gastroenterology. 2019;157:227–41.
- Gane E, et al. J Hepatol. 2019;71:900–7.
- Janssen H, et al. AASLD 2020:Abstract 0829.
- Zoulim F, et al. Hum Vaccin Immunother . 2020;16:388–99.
- Jansen DT, et al. Clin Trans Immunology. 2021;10:e1232.
- Hasanpourghadi M, et al. EASL 2021:Abstract OS-2478.
- Lee J, et al. For Immunopathol Dis Therap.. 2015;6:7–17.
- Le Bert N, et al. Gastroenterology. 2020;159;2:652–64.
- Rivino L, et al. J Clin Invest. 2018;128:668–81.
- Cornberg M, et al. J Hepatol. 2017;66:398–411.
- Hasanpourghadi M, et al. EASL 2020:Abstract 1303.
- Virion Therapeutics. Data on file.
- Hasanpourghadi M, et al. APASL 2022:Abstract OS-0685.
- Luber A, et al. EASL 2023. Poster #TOP-107.